More than 300 million people live with rare diseases around the world. What makes a disease rare? The definition varies, but many organizations consider a condition rare if it affects fewer than 1 in 2000 people. Recent years have seen a surge of interest in rare diseases, driven by advances in technology, regulatory policy changes, and growing competition in traditional disease areas. As a result, many life sciences firms are exploring the unmet needs of over 8000 rare diseases, leading to a growing market opportunity.
Rare diseases, common journeys
While there is large variation among rare diseases, people living with a rare disease face common challenges.
- Diagnosis is a long and difficult process: Many rare diseases take years to be properly diagnosed, leading to delays in treatment and significant physical, emotional, and financial burdens for patients.
- Limited care and support options: Rare diseases are often poorly understood, even by many healthcare professionals, leading to poor patient access to information and support.
- Financial burden: Treatment can be extremely expensive, with costly medications and other medical interventions.
- Social and psychological stress: Patients can feel alone or unsupported due to the lack of awareness and understanding of their condition.
Improvement requires data
Access to sufficient data is a major barrier to advancing knowledge and treatment for rare diseases. Due to their low prevalence, finding patients is challenging, and the lack of data leads to wide variation in regulatory approval and reimbursement for treatments between countries. As a result, real-world data (RWD) is becoming increasingly important for improving outcomes for rare diseases. It can be used to better design and execute clinical trials as well as offering valuable insights into disease progression, patient characteristics and outcomes.
Another key benefit of RWD is its potential in better understanding treatment patterns across different jurisdictions. This insightful approach relies exclusively on the ability to examine real-world patient journeys in one market, understand treatment patterns for a particular condition, and then compare findings to practices in other global markets.
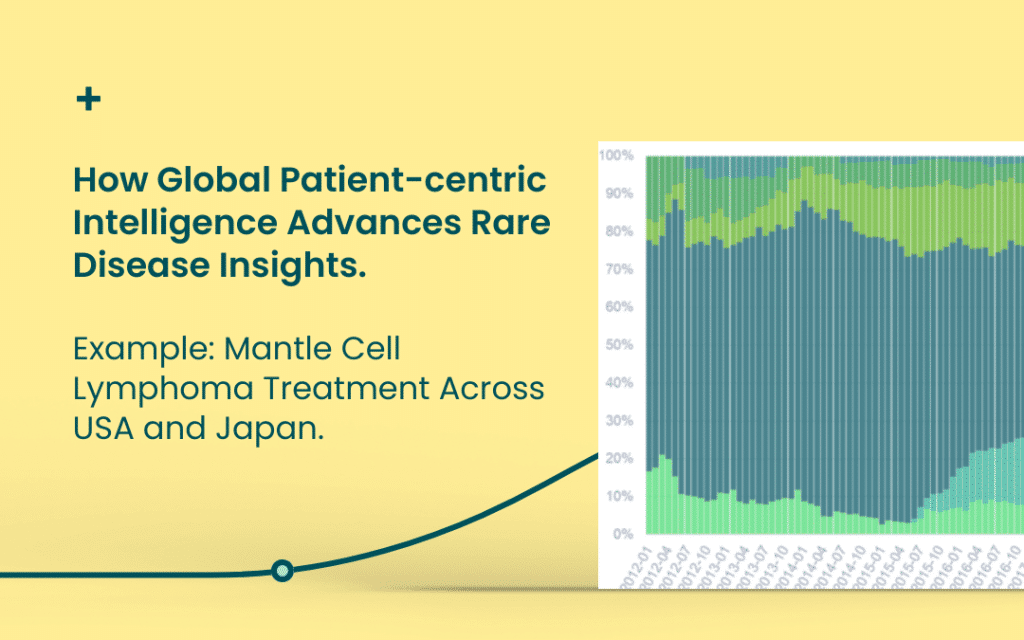
RWD in action – a mantle cell lymphoma example
Mantle cell lymphoma is a rare form of non-Hodgkin’s lymphoma affecting a few people per million annually. While there is no cure for MCL, treatment can help prolong survival and improve quality of life. The challenge is that there is no clear consensus on the right treatment for each patient. The preferred treatment approach for patients with MCL often varies from doctor to doctor, country to country. At Prospection, where we have RWD from multiple countries, we see differences in treatment patterns among Japan and the United States.
Frontline setting
In Japan and the US, Bendamustine-rituximab (B-R) is the dominant regimen used in the frontline setting, in line with evidence that it is less toxic and produces higher response rates compared to patients using rituximab and conventional chemotherapy. Additionally, in the US, targeted therapies like ibrutinib, bortezomib, and acalabrutinib are also widely used in the frontline setting.
In Japan, frontline treatment typically involves B-R, rituximab and ibrutinib
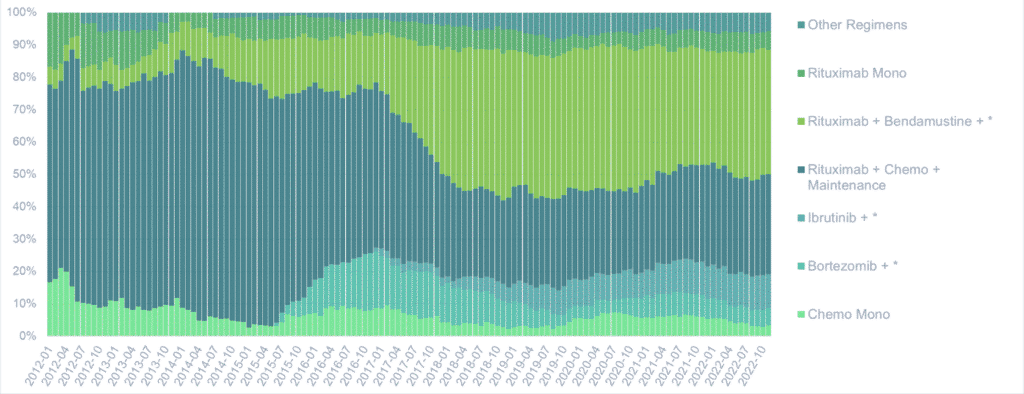
In the US, targeted therapies such as bortezomib, ibrutinib and acalabrutinib are frequently used

Second-line treatment
Therapy for MCL is not curative and virtually all patients will relapse. As in the frontline setting, we see differences in treatment patterns across countries.
In the US, second-line therapy differs from Japan with rituximab-based therapy accounting for the lion’s share of the market. Targeted therapies are less often used in relapse in the US as compared to Japan. As is common with rare disease data, these trends need to be interpreted with caution due to the small sample sizes of each cohort.
The US market shows higher usage of rituximab based therapies in the second line as compared to Japan
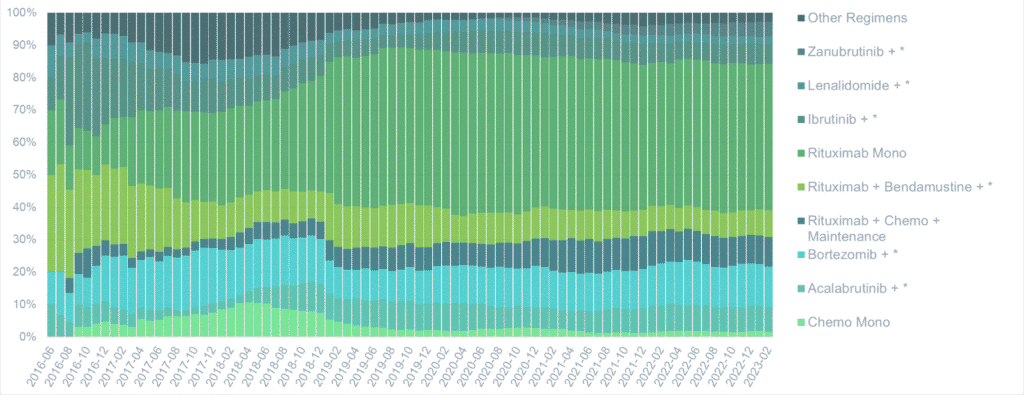
A number of treatment regimens have been evaluated in patients with relapsed MCL, but evidence to guide treatment decisions remains elusive. Currently, treatment choices are primarily made based on the patient’s prior treatment, patient comorbidities and performance status, the regimens’ expected toxicities, and the clinician’s previous experience prescribing the regimens.
RWD offers a path forward
The effect of these differences in access and treatment patterns with respect to patient and health system outcomes remains to be seen. Further research is required. However, we know that barriers to accessing best-practise care, whether because of variability in insurance coverage and/or reimbursement decisions, can result in critical delays to treatment, and lead patients to go without treatment or use sub-optimal alternatives. According to the National Organization for Rare Disorders (NORD) 2019 survey, 61% of patients had been denied or faced delays accessing treatments that required pre-approval from an insurance company.
Understanding treatment patterns using real world data from multiple countries can help mobilize the real world evidence needed to influence care pathways, inform policy and advocacy, strengthen regulatory process, bolster sample sizes for research, and augment traditional research methodologies. As rare diseases such as MCL affect small numbers of people in each country, an international view incorporating data from multiple countries and pooling of patients from different datasets could greatly speed up progress for rare diseases.
Bringing hope for people living with rare diseases
Each year a greater number of new rare diseases treatments are approved globally. At every stage, more robust evidence derived from RWD is emerging to guide decision making at all levels of care.
This RWD is core to ensuring patients with rare disease can access the right treatment at the right time. The capability to use RWD from multiple countries strengthens the fight, contributing to better ways to develop and deploy effective therapies.
By raising awareness and advocating for rare disease research, better outcomes for patients and families affected by these challenging conditions are in reach. Visit the Rare Diseases Day website to learn more about rare diseases and find out about how you can get involved with an event near you.
If you’d like to learn more about how Prospection works with patient-cntric intelligence to help pharma companies improve patient outcomes, contact us today.